FDA breakthrough therapy designation1
FDA breakthrough therapy designation1
First-in-class, long-acting HIV-1 capsid inhibition with SUNLENCA®2,3
BREAKING
BARRIERS
in HIV treatment™
Help heavily treatment-experienced (HTE) adults
break away from HIV-1 treatment failures by adding
SUNLENCA,* a novel HIV-1 capsid inhibitor2
SUNLENCA should always be taken with an optimized background regimen.
Actor portrayal.
Actor portrayal.
Help heavily treatment-experienced (HTE)
adults break away from HIV-1 treatment
failures by adding SUNLENCA,* a novel
HIV-1 capsid inhibitor2
SUNLENCA should always be taken with an optimized background regimen.
Important Safety Information
Contraindications
- Coadministration: Concomitant administration of SUNLENCA is contraindicated with strong CYP3A inducers.
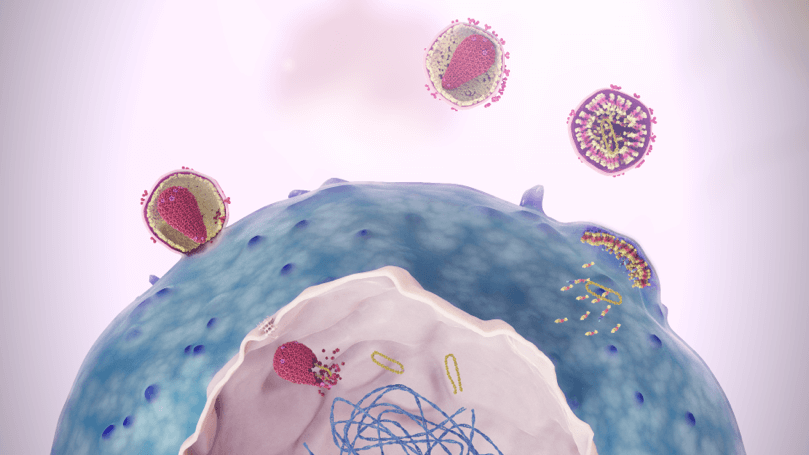
A novel, first-in-class
capsid inhibitor1-3
SUNLENCA received FDA breakthrough therapy designation and has a novel MOA that inhibits capsid function at multiple stages of the HIV-1 life cycle.
Watch a video to see the role the capsid protein plays in HIV-1 replication and how SUNLENCA targets these essential functions.
Click for Important Safety Information for SUNLENCA.
Demonstrated potent antiviral activity2,5
See the data from CAPELLA, a 52-week, partially randomized, placebo-controlled, double-blind, multicenter trial studying the efficacy and safety of SUNLENCA® in HTE HIV-1 patients with multidrug resistance (N=72).
88% of patients taking SUNLENCA (n=24) achieved the primary endpoint, the proportion of patients in the randomized cohort achieving ≥0.5 log10 copies/mL reduction from baseline in HIV-1 RNA at Day 15, vs 17% of patients taking placebo (n=12).
Adverse reactions
- Most common adverse reactions (incidence ≥3%, all grades) are injection site reactions (65%) and nausea (4%).
Actor portrayal.

Administered only twice a year In Addition to an OBR2,*
SUNLENCA®, when combined with an optimized background regimen (OBR), offers HTE adults a long-acting treatment option.
Prior to starting every-6-month subcutaneous injections, patients should complete an initiation regimen listed in the dosing section.
Missed Dose: During the maintenance period, if more than 28 weeks have elapsed since the last injection and if clinically appropriate to continue SUNLENCA treatment, restart the initiation dosage regimen from Day 1, Option 1 or Option 2.