This information is intended for US healthcare professionals.
A complete HIV-1 treatment regimen that includes SUNLENCA also requires an optimized background regimen1
According to the DHHS guidelines, for multiple or extensive drug resistance with few treatment options, a new regimen should include at least 2, and preferably 3, fully active agents (including those with novel mechanisms of action). If choosing a regimen with fewer than 3 fully active drugs, include as many fully active drugs as possible, along with potentially partially active drugs.2
Prior to starting SUNLENCA, healthcare providers should carefully select patients who agree to the required every-6-month injection dosing schedule and counsel patients about the importance of adherence to scheduled SUNLENCA dosing visits and concomitant oral antiretroviral therapy to help maintain viral suppression and reduce the risk of viral rebound and potential development of resistance with missed doses.1
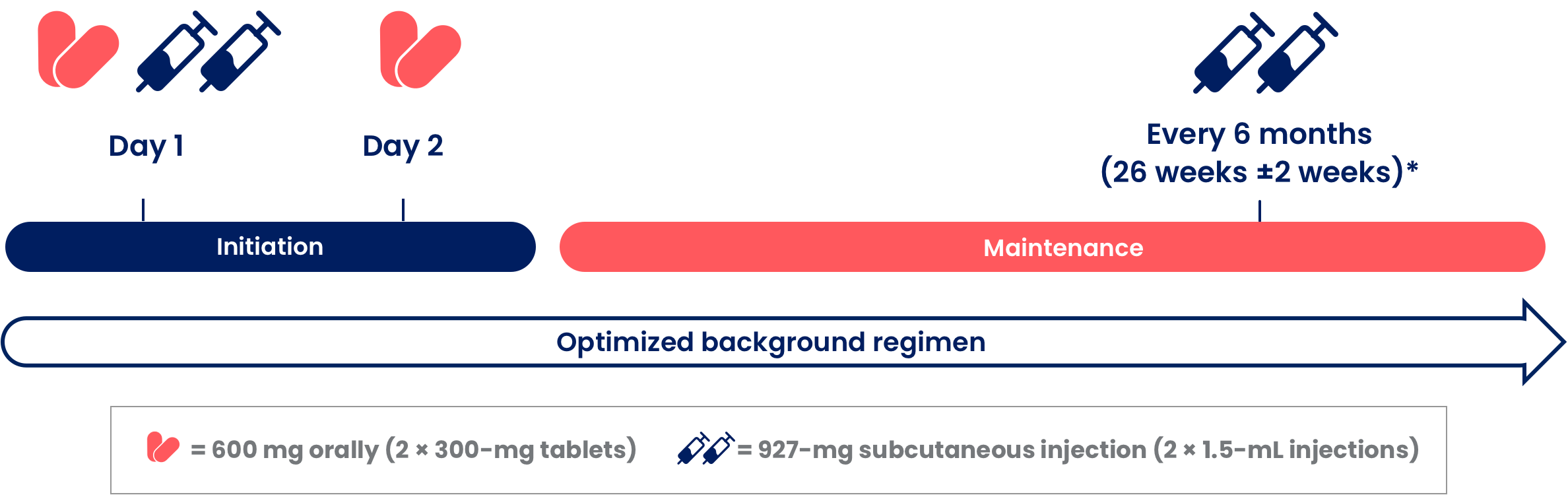
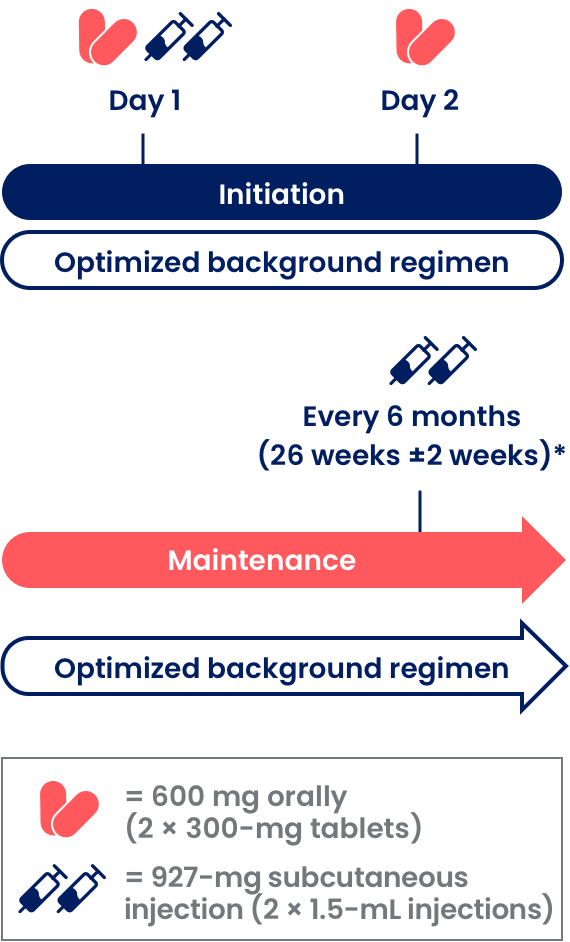
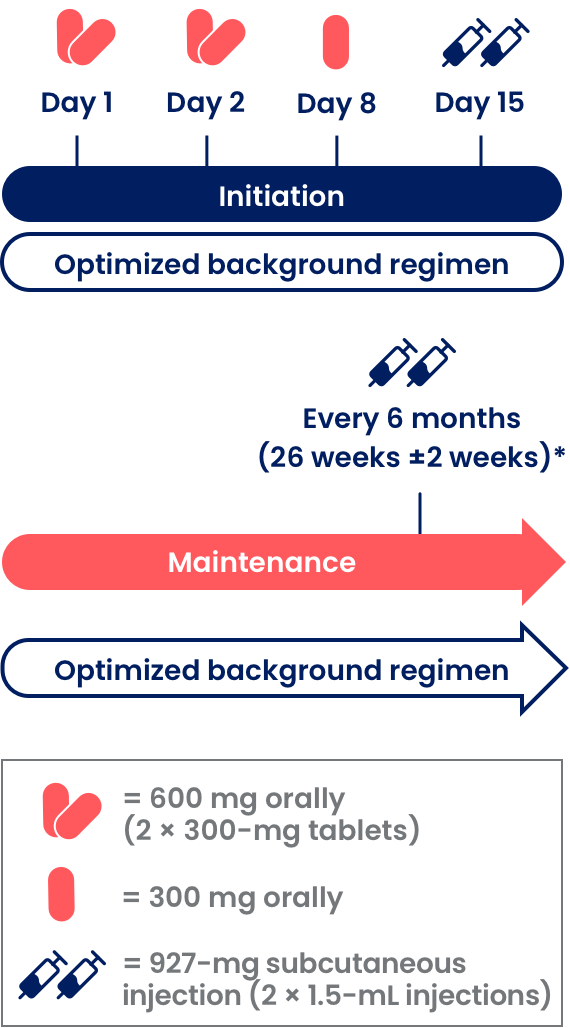
Two ways to start SUNLENCA
Oral initiation allows therapeutic drug levels to be reached quickly. A treatment regimen incorporating SUNLENCA begins with 1 of the 2 initiation options, based upon your assessment of which is appropriate for your patient.1,3
Initiation Option 1 (Day 1 First SC Injection)
Initiation Option 2 (Day 15 First SC Injection)
Dosing Regimen
Initiation Option 1 (Day 1 First SC Injection)1


Dosing Regimen
Initiation Option 2 (Day 15 First SC Injection)1

From the date of the last injection.
Missed dose information
Planned missed injections1
During the maintenance period, if a patient plans to miss a scheduled 6-month injection visit by more than 2 weeks, SUNLENCA tablets may be taken for up to 6 months until injections resume.
At 26 to 28 weeks since the last injection, patients should begin taking the maintenance oral dosage of 300 mg, taken once every 7 days for up to 6 months. Maintenance injections should resume within 7 days of the last oral dose.
Unplanned missed injections1
Patients who miss a scheduled injection visit should be clinically reassessed, including consideration of lenacapavir resistance testing, to ensure resumption of therapy remains appropriate. During the maintenance period, if more than 28 weeks have passed since the last injection and SUNLENCA tablets have not been taken, restart the initiation dosage regimen from Day 1, using Option 1 or Option 2, and then continue with maintenance injection dosing. Adherence to the injection dosing schedule is strongly recommended.
ADDING EVERY-6-MONTH subcutaneous injections of SUNLENCA TO AN OBR provides HTE ADULTS WITH A LONG-ACTING TREATMENT OPTION1
Storage and handling of SUNLENCA1
SUNLENCA tablets
SUNLENCA injection
SUNLENCA, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 whose current antiretroviral regimen is failing due to resistance, intolerance, or safety considerations.
Contraindications
Warnings and precautions
SUNLENCA, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 whose current antiretroviral regimen is failing due to resistance, intolerance, or safety considerations.
Tap for Important Safety Information
Contraindications
Warnings and precautions
SUNLENCA, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 whose current antiretroviral regimen is failing due to resistance, intolerance, or safety considerations.